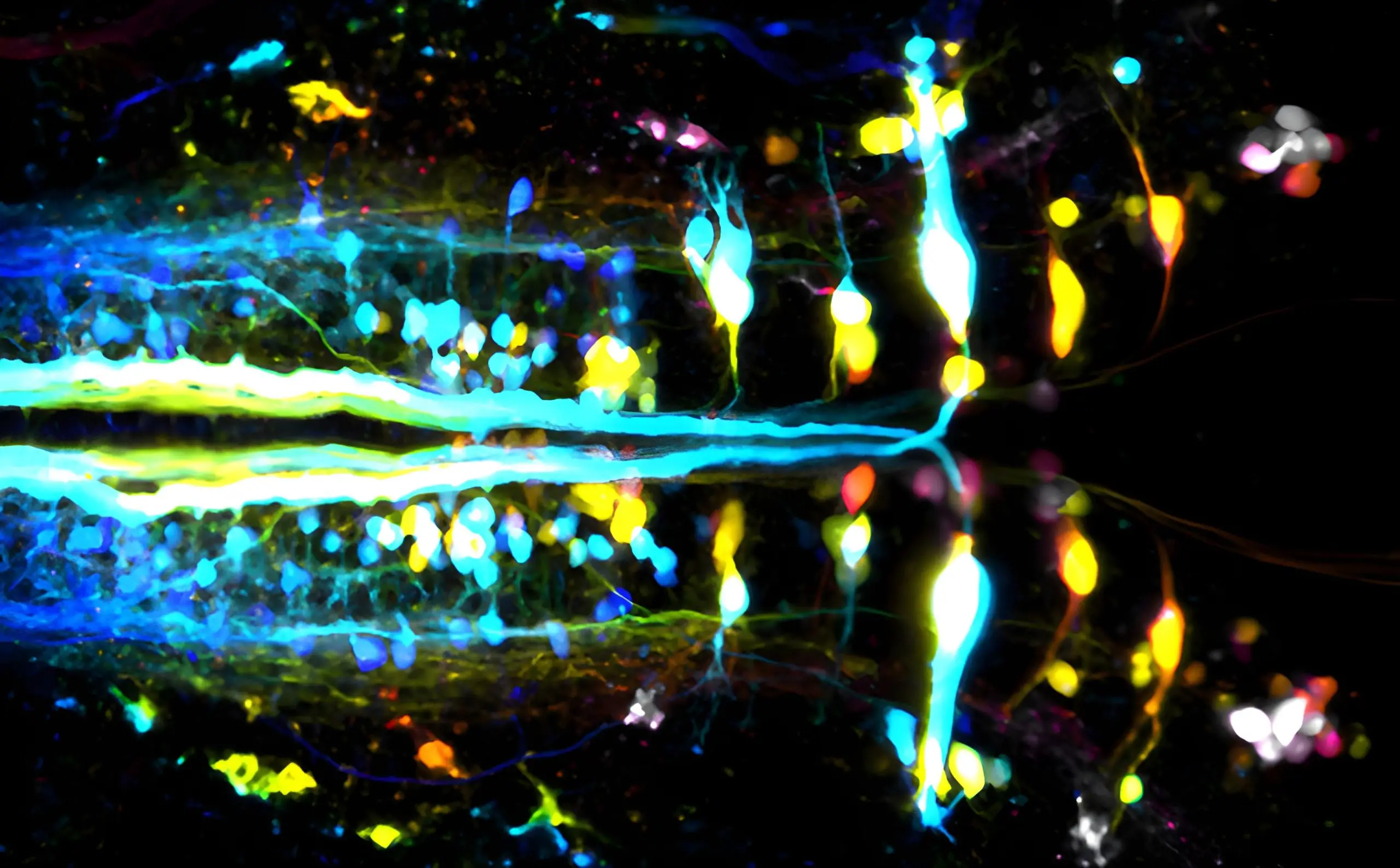
Paris Brain Institute researchers have explored the complexities of walking, emphasizing the mesencephalic locomotor region’s role in movement. Their findings, based on zebrafish studies, hold potential implications for understanding diseases like Parkinson’s. (Corticospinal neurons in zebrafish.) Credit: Martin Carbo-Tano
Walking is a complex mechanism involving both automatic processes and conscious control. Its dysfunction can have multiple, sometimes extremely subtle causes, within the motor cortex, brain stem, spinal cord, or muscles. At Paris Brain Institute, Martin Carbo-Tano, Mathilde Lapoix, and their colleagues in the “Spinal Sensory Signaling” team, led by Claire Wyart (Inserm), have focused on a specific component of locomotion: forward propulsion.
In a study published on September 4 in Nature Neuroscience, they show that it involves a region classically called the mesencephalic locomotor region, which controls the vigor and speed of movement, and transmits the nervous message to the spinal cord via control neurons located in the brainstem. This new mapping carried out in zebrafish corroborates recent studies in mice. It could eventually be extended to humans—helping to understand how movement control circuits can malfunction, such as in Parkinson’s disease.
Complexity Beneath Routine Movement
For those fortunate enough to walk normally, wandering is such an expected behavior that we hardly consider that it involves complex, partly involuntary processes. “Animals move to explore their environment in search of food, interaction with others, or simply out of curiosity. But the perception of danger or a painful stimulus can also activate an automatic flight reflex,” Martin Carbo-Tano, a post-doctoral fellow at Paris Brain Institute, explains.
In both cases, movement initiation relies on the activation of so-called reticulospinal control neurons, which form an intertwined network in the most posterior part of the brain—the brainstem. These neurons relay nerve signals between the brain and the spinal cord and are essential for motor control of the limbs and trunk and movement coordination.
Upstream of the reticulospinal neurons is the mesencephalic locomotor region (MLR), which is also essential for locomotion since, in animals, its stimulation triggers forward propulsion. It is found in many vertebrates, including monkeys, guinea pigs, cats, salamanders, and even lampreys.
“Because the role of the MLR is conserved in many vertebrate species, we assume that it is an ancient region in their evolution—essential for initiating walking, running, flying, or swimming,” he adds. “But until now, we didn’t know how this region transmits information to the reticulospinal neurons. This prevented us from gaining a global view of the mechanisms that enable the vertebrae to set themselves in motion and, therefore, from pointing out possible anomalies in this fascinating machinery.”
Innovations in Locomotion Study
Studying movement initiation is a little tricky: neurons located in the brain stem are not easily accessible and observing their activity in vivo in a moving animal proved difficult. To solve this problem, Martin Carbo-Tano has developed a new approach to stimulate tiny areas in the brain.
Together with Mathilde Lapoix, a Ph.D. student in Claire Wyart’s team at Paris Brain Institute, the researchers took advantage of the transparency of the zebrafish larvae brain to localize the structures involved in locomotion downstream of the MLR and follow the propagation of nerve impulses. This method, inspired by the work of their collaborator Réjean Dubuc at Montréal University, allowed them to make several remarkable discoveries.
“We observed that neurons in the mesencephalic locomotor region are stimulated when the animal moves spontaneously, but also in response to a visual stimulus. They project through the pons—the central part of the brain stem—and the medulla to activate a subpopulation of reticulospinal neurons called ‘V2a’. These neurons control the finer details of movement, such as starting, stopping, and changing direction. In a way, they give steering instructions! Previous work on mice had revealed that reticulospinal neurons control turning; Martin and Mathilde have discovered the control circuit that triggers forward locomotion,” Claire Wyart says.
The Midbrain, a Concentration of Intensity
To better understand the effects of this mechanism on the movements of larval zebrafish, the researchers triggered it experimentally by stimulating the mesencephalic locomotor region. They observed that the duration and vigor of forward movement correlated with the intensity of the stimulation.
“Quadrupeds can adopt different gaits, such as walking, trotting, or galloping. But aquatic animals also mark gait transitions,” Martin Carbo-Tano adds. “We think that MLR has a role to play in this intensification of movement, which we have observed in zebrafish.”
Implications and Future Directions
For the first time, this work made it possible to map the neuronal circuits involved in initiating forward movement—a deficient function in patients with Parkinson’s disease. This is an essential step in shedding light on the motor control mechanisms upstream of the spinal cord.
One day, it may be possible to identify and control all the reticulospinal neurons one by one to model in detail the workings of locomotion and repair those that do not function correctly.
Reference: “The mesencephalic locomotor region recruits V2a reticulospinal neurons to drive forward locomotion in larval zebrafish” by Martin Carbo-Tano, Mathilde Lapoix, Xinyu Jia, Olivier Thouvenin, Marco Pascucci, François Auclair, Feng B. Quan, Shahad Albadri, Vernie Aguda, Younes Farouj, Elizabeth M. C. Hillman, Ruben Portugues, Filippo Del Bene, Tod R. Thiele, Réjean Dubuc and Claire Wyart, 4 September 2023, Nature Neuroscience.
DOI: 10.1038/s41593-023-01418-0
This project has benefited from the European Research Council (ERC), the Foundation for Medical Research (FRM), the Bettencourt-Schueller Foundation (FBS), the Marie Skłodowska-Curie European Training Network program, funded under Horizon 2020, the New York Stem Cell Foundation (NYSCF) and the National Institute of Health (NIH).