Compared with the purified protein in vitro, intracellular biological macromolecules present a more complete and physiological conformation. Therefore, studying the three-dimensional structure of protein directly in vivo is the next goal of structural biology and also the frontier of cryo-electron microscopy (cryo-EM) technology development.
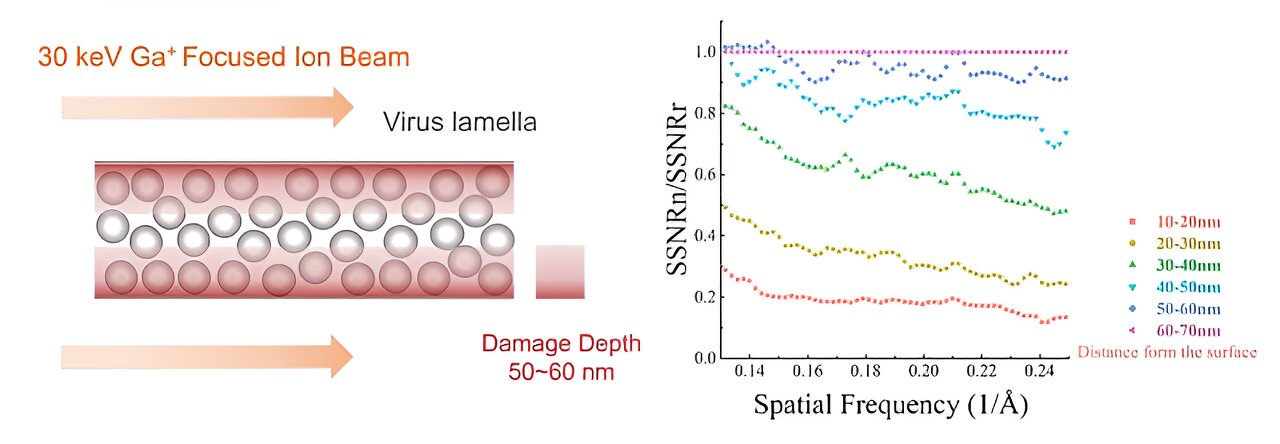
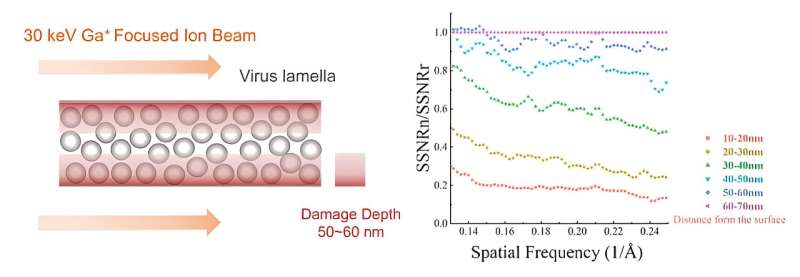
Cells need to be thinned into ~150 nm lamellae to meet the thickness requirement of cryo-EM imaging, and a focused ion beam (FIB) is used to produce the qualified lamellae by milling the frozen cell with high-energy ion beams. However, FIB damages the lamellae during milling at both the upper and lower surfaces, and a quantitative experimental analysis of the damage has yet to be done and how to reduce the damage is unknown.
In a study published in Structure, researchers from the Institute of Biophysics of the Chinese Academy of Sciences presented a quantitative analysis method for cryo-FIB damage and demonstrated that low-energy beam polishing was essential for producing high-quality thinner lamellae.
This work establishes a new method for quantifying the damage of FIB to cellular lamellae. The proteins in the cellular lamellae are grouped according to the distance from the surfaces by the electron tomography reconstruction. Based on the 3D reconstruction of proteins in each group, researchers calculated the average signal-to-noise ratio (SNR) of protein in each group. The average SNRs reflected the data quality of protein in different groups and also represented the damage degree of the FIB to the protein at different distances from the surfaces quantificationally.
The research found that FIB caused non-negligible damage at a depth of 50 nm on the surface of lamellae. The damage on the surface was similar to that from exposure to 16 e–/Å2 in a 300-kV cryo-electron microscope and the damage was 3.5 e–/Å2 at ~50 nm region. The results meant that when the thickness of lamella is less than 100 nm, the damage from the both surfaces will be doubled in the center region, resulting in damage greater than 7 e–/Å2 pre-exposure.
Theoretically, to reach higher 3D reconstruction resolution, the thickness of the cell lamella should be as thin as possible. However, FIB will damage all the proteins in the lamella with thickness less than 100 nm. Such a contradiction will make it difficult to obtain the 3D reconstruction with near-atomic resolution for the FIB-milled lamellae. In order to break the limitation of the damage depth on the minimum thickness of the lamella, further research found that the damage layer thickness can be reduced to less than 30 nm by reducing the milling voltage of the FIB to 8 keV.
The improvement of resolution is essential for the study of protein in situ, and obtaining high-quality lamellae is an important precondition for improving the resolution of 3D reconstruction. This work not only provided a tool for qualitatively measuring lamellae quality, but also proposed a scheme for reducing FIB damage in cryo-lamellae, which is conducive to further improvement of the resolution of in-situ structure analysis.
More information:
Qi Yang et al, The reduction of FIB damage on cryo-lamella by lowering energy of ion beam revealed by a quantitative analysis, Structure (2023). DOI: 10.1016/j.str.2023.07.002
Provided by
Chinese Academy of Sciences
Citation:
Novel method reveals energy-dependent radiation damage to milled lamella by focused ion beam (2023, August 1)
retrieved 2 August 2023
from https://phys.org/news/2023-08-method-reveals-energy-dependent-milled-lamella.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

