

The Rosetta orbiter spectrometer for ion and neutral analysis (ROSINA) instrument orbited comet 67P to revolutionize our understanding of cometary material composition. A key finding of the satellite was to explore the composition of comet 67P/Churyumov-Gerasimenko. In a new report published in Science Advances, Ahmed Mahjoub and a team of planetary scientists in the Jet Propulsion Lab at CalTech, the Space Science Institute Colorado, and the University of Bern in Switzerland, used the ROSINA data to study dust particles volatilized during a dust event in September 2016.
The scientists reported the detection of large organosulfur species, on the comet’s surface. They then conducted laboratory simulations to indicate the formation of this material from chemical reactions initiated by irradiating mixed ices containing hydrogen sulfide. The results highlighted the significance of cometary sulfur chemistry and its presence in precometary materials to facilitate the detection of organosulfur materials in other comets and icy small bodies by using the James Webb Space Telescope.
Landing on a comet
When the Rosetta mission visited comet 67P, the satellite revealed remarkable insights to the diverse molecules on the comet. The researchers detected organics by using a remote sensing instrument, visible and infrared thermal imaging spectrometry, and a series of instruments including ROSINA, Ptolemy and the cometary sampling and composition experiment. The measurements made using ROSINA provided substantial information of the complex organic chemistry in cometary materials, alongside further insights to the composition of the semi-volatile phases of comet 67P.
The measurements further revealed the detection of ammonium salts. In this work, Mahjoub and colleagues discussed the data gathered from the Rosetta probe and ROSINA during an event of enhanced dust impact on the instrument. They interpreted the data to reveal the presence of large organosulfur molecules with low volatility embedded in the dust grains of comet 67P. The team completed in-lab simulations of the organic chemistry that began on the comet due to irradiation of simple ice mixtures in the presence or absence of hydrogen sulfide. The outcomes indicated the dominance of sulfur in the environment, and a possible ice-chemistry origin to the sulfur-bearing species on the cometary material.
The dust event
Prior to landing on the comet, Rosetta flew elliptical orbits during the last few weeks of its mission with the pericenter altitude gradually lowered. On September 2016, the spacecraft reached its closest distance from the comet. It is assumed that the space probe was hit by a chunk of ice or dust prior to that, which led to the observation of high-density gas plumes for about 3 hours in the vicinity of the instrument.
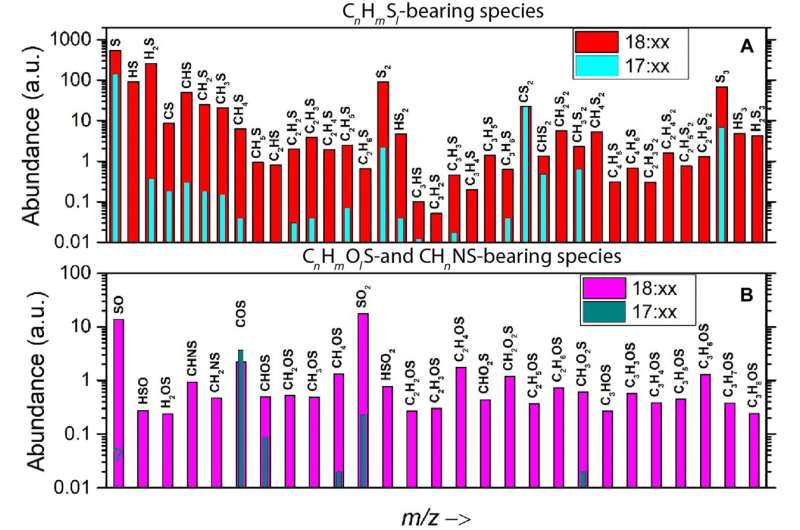
The measurements made during the study indicated the abundance of a variety of sulfur-bearing molecules, prior to and after the dust event. The team conducted mass spectrometry measurements to identify carbonyl sulfide and carbon disulfide as species that did not significantly increase during the event due to their higher volatility, when compared with sulfur dioxide, which increased by about two orders of magnitude. The team further monitored the presence of semi-volatile organo-sulfurous molecules on the surface of comet 67P.
Simulations in the lab
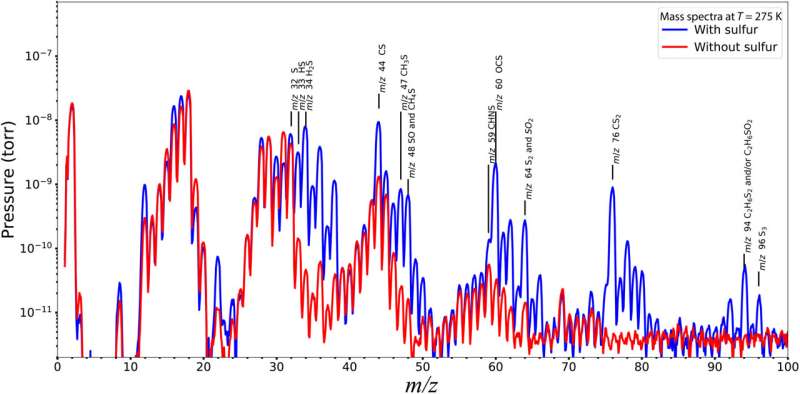
The ROSINA-double focusing mass spectrometer (ROSINA-DFMS) data obtained during the dust event showed sulfur chemistry to be more complex and diverse than hitherto known or assumed via measurements in the undisturbed coma of the comet. Mahjoub and colleagues assumed this outcome to have resulted from the ice chemistry involving hydrogen sulfide. To explore this in the lab, the team performed electron irradiation experiments on ice mixtures in the presence or absence of the molecules.
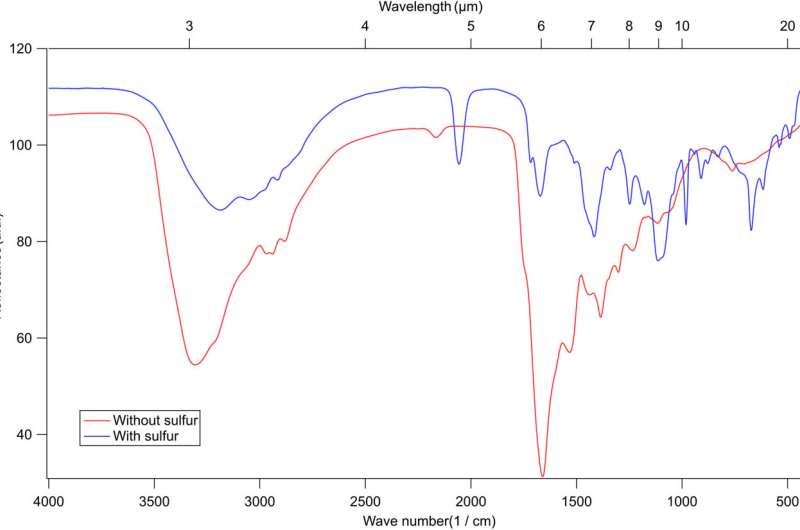
The experimental setup included a high-vacuum stainless-steel chamber, where the team deposited ices on a gold substrate attached to a cold finger of a helium cryostat through a gas, to prepare gas mixtures. The setup included an electron gain in the chamber and a Faraday cup to monitor the electron beam current. The team detected the evolving samples with a Fourier transform infrared spectrometer. Further experiments highlighted the rapid dissociation of hydrogen sulfide in the setup, compared to methanol and water samples used in similar experiments, to produce a high concentration of reactive sulfur bearing radicals to predominantly affect the chemistry in the ice films.
Outlook
In this way, Ahmed Mahjoub and colleagues characterized organic heteropolymers in small interstellar icy grains and icy bodies. They assumed hydrogen sulfide ice chemistry to be likely for the observed species. They highlighted the presence of other pathways to form organosulfur compounds in the diffuse interstellar medium, and in the solar nebula. Using in-lab simulations, the scientists showed that sulfur-bearing organic compounds could be formed via sulfur ion bombardment of astrophysical ices containing carbon, oxygen, and nitrogen constituents.
The James Webb Space Telescope incorporated during this work can increase the understanding of the chemistry of the solar system, including comets, and asteroids. This instrument can also assist researchers to unveil the composition of a variety of such interstellar bodies alongside their similarities or differences, to understand the formation and evolution of the solar system; where sulfur chemistry is of interest. The fate of sulfur has a key role in the evolution of comets and interstellar icy bodies, although much of its role in the building blocks of the solar system remains to be known. The element, however, holds promising capacity to answer the origin and evolution of such icy small bodies.
More information:
Ahmed Mahjoub et al, Complex organosulfur molecules on comet 67P: Evidence from the ROSINA measurements and insights from laboratory simulations, Science Advances (2023). DOI: 10.1126/sciadv.adh0394
I. P. Wright et al, CHO-bearing organic compounds at the surface of 67P/Churyumov-Gerasimenko revealed by Ptolemy, Science (2015). DOI: 10.1126/science.aab0673
© 2023 Science X Network
Citation:
Complex organosulfur molecules on comet 67P: Evidence from Rosetta orbiter and the lab (2023, June 30)
retrieved 30 June 2023
from https://phys.org/news/2023-06-complex-organosulfur-molecules-comet-67p.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.