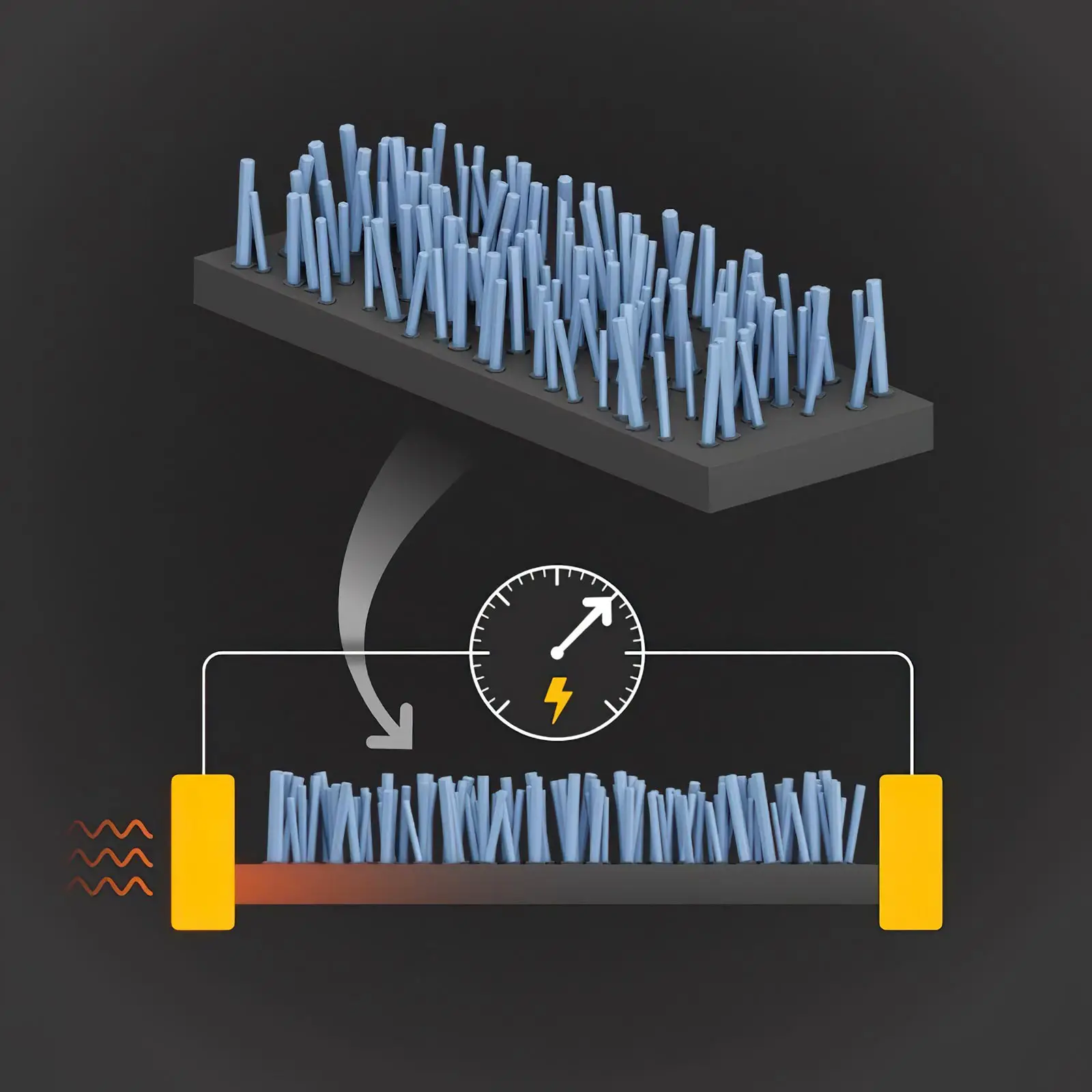
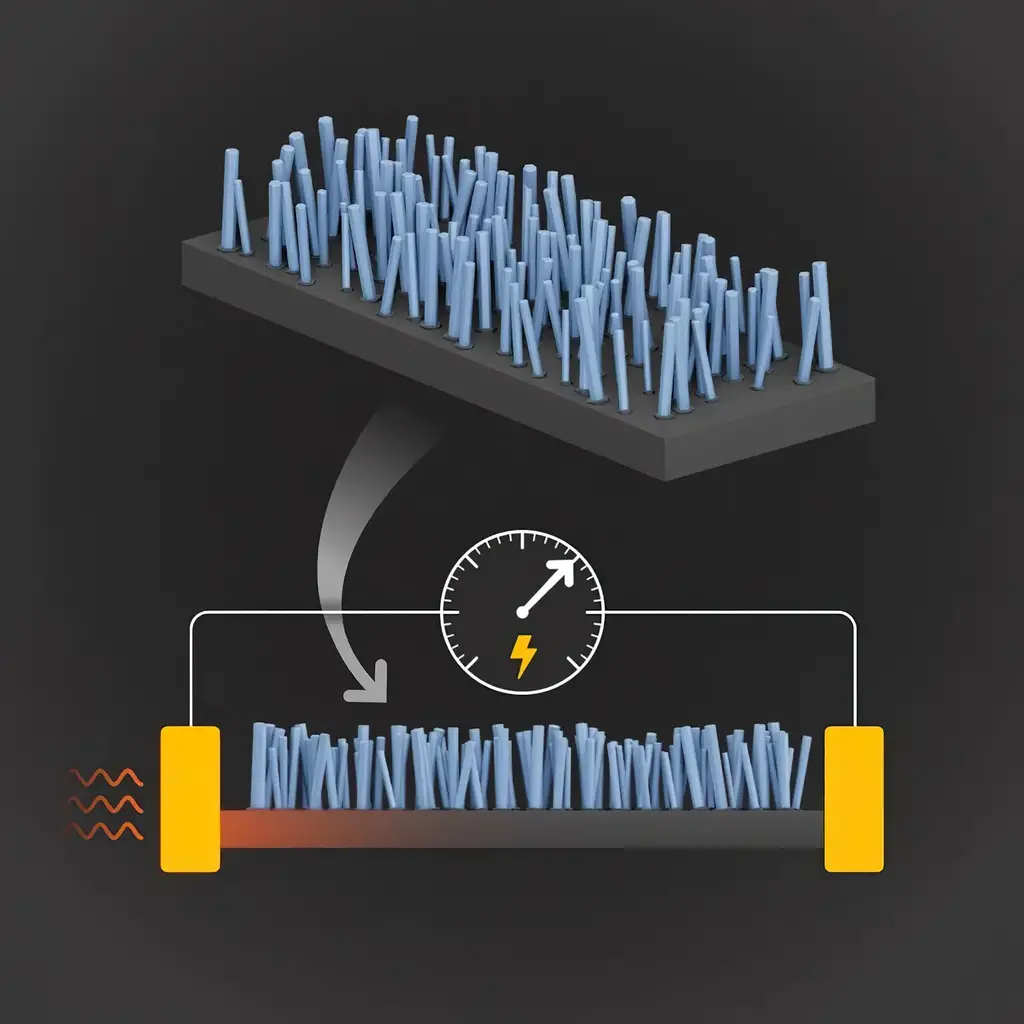
Illustration of nanopillars used in a new design to efficiently convert heat energy into electricity. Credit: S. Kelley/NIST
A team from NIST and the University of Colorado Boulder have developed a novel device using gallium nitride nanopillars on silicon that significantly improves the conversion of heat into electricity. This could potentially recover large amounts of wasted heat energy, benefiting industries and power grids.
Researchers at the National Institute of Standards and Technology (NIST) have fabricated a novel device that could dramatically boost the conversion of heat into electricity. If perfected, the technology could help recoup some of the heat energy that is wasted in the U.S. at a rate of about $100 billion each year.
The new fabrication technique — developed by NIST researcher Kris Bertness and her collaborators — involves depositing hundreds of thousands of microscopic columns of gallium nitride atop a silicon wafer. Layers of silicon are then removed from the underside of the wafer until only a thin sheet of the material remains. The interaction between the pillars and the silicon sheet slows the transport of heat in the silicon, enabling more of the heat to convert to electric current. Bertness and her collaborators at the University of Colorado Boulder recently reported the findings in the journal Advanced Materials.
Once the fabrication method is perfected, the silicon sheets could be wrapped around steam or exhaust pipes to convert heat emissions into electricity that could power nearby devices or be delivered to a power grid. Another potential application would be cooling computer chips.
By growing nanopillars above a silicon membrane, NIST scientists and their colleagues have reduced heat conduction by 21% without reducing electrical conductivity, a result that could dramatically boost the conversion of heat energy into electrical energy. In solids, heat energy is carried by phonons, periodic vibrations of atoms in a crystal lattice. Certain vibrations of the phonons in the membrane resonate with those in the nanopillars, acting to slow the transfer of heat. Crucially, the nanopillars do not slow the movement of electrons, so that electrical conductivity remains high, creating a superior thermoelectric material. Credit: S. Kelley/NIST
The NIST-University of Colorado study is based on a curious phenomenon first discovered by German physicist Thomas Seebeck. In the early 1820s, Seebeck was studying two metal wires, each made of a different material, that were joined at both ends to form a loop. He observed that when the two junctions connecting the wires were kept at different temperatures, a nearby compass needle deflected. Other scientists soon realized that the deflection occurred because the temperature difference induced a voltage between the two regions, causing current to flow from the hotter region to the colder one. The current created a magnetic field that deflected the compass needle.
In theory, the so-called Seebeck effect could be an ideal way to recycle heat energy that would otherwise be lost. But there’s been a major obstacle. A material must conduct heat poorly in order to maintain a temperature difference between two regions yet conduct electricity extremely well to convert the heat to a substantial amount of electrical energy. For most substances, however, heat conductivity and electrical conductivity go hand in hand; a poor heat conductor makes for a poor electrical conductor and vice versa.
In studying the physics of thermoelectric conversion, theorist Mahmoud Hussein of the University of Colorado discovered that these properties could be decoupled in a thin membrane covered with nanopillars — standing columns of material no more than a few millionths of a meter in length, or about one-tenth the thickness of a human hair. His finding led to the collaboration with Bertness.
Using the nanopillars, Bertness, Hussein and their colleagues succeeded in uncoupling the heat conductivity from electrical conductivity in the silicon sheet — a first for any material and a milestone for enabling efficient conversion of heat to electrical energy. The researchers reduced the heat conductivity of the silicon sheet by 21% without lowering its electrical conductivity or changing the Seebeck effect.
In silicon and other solids, atoms are constrained by bonds and cannot move freely to transmit heat. As a consequence, the transport of heat energy takes the form of phonons — moving collective vibrations of the atoms. Both the gallium nitride nanopillars and the silicon sheet carry phonons, but those within the nanopillars are standing waves, pinned down by the walls of the tiny columns much the way a vibrating guitar string is held fixed at both ends.
The interaction between the phonons traveling in the silicon sheet and the vibrations in the nanopillars slow the traveling phonons, making it harder for heat to pass through the material. This reduces the thermal conductivity, thus increasing the temperature difference from one end to the other. Just as importantly, the phonon interaction accomplishes this feat while leaving the electrical conductivity of the silicon sheet unchanged.
The team is now working on structures fabricated entirely of silicon and with a better geometry for thermoelectric heat recovery. The researchers expect to demonstrate a heat-to-electricity conversion rate high enough to make their technique economically viable for industry.
Reference: “Semiconductor Thermal and Electrical Properties Decoupled by Localized Phonon Resonances” by Bryan T. Spann, Joel C. Weber, Matt D. Brubaker, Todd E. Harvey, Lina Yang, Hossein Honarvar, Chia-Nien Tsai, Andrew C. Treglia, Minhyea Lee, Mahmoud I. Hussein and Kris A. Bertness, 23 March 2023, Advanced Materials.
DOI: 10.1002/adma.202209779
This research was funded in part by the Department of Energy’s Advanced Research Projects Agency-Energy.