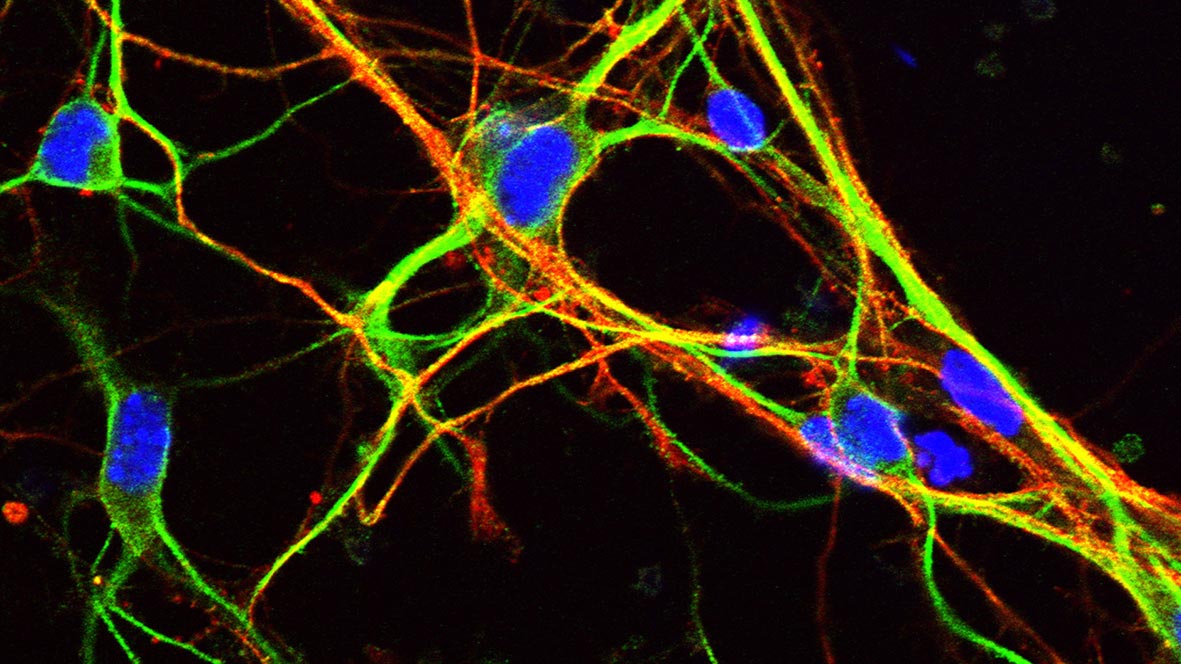

Differentiated cortical neurons expressing the axonal marker Tau (green) and the dendritic marker MAP-2 (red). Credit: Dr. Robert Williams, University of Bath
Scientists are starting to understand the precise workings of a type of gene that, unlike other genes, does not code for proteins – the building blocks of life.
New research shows the mechanism by which genes coding for a subset of long non-coding RNA (lncRNA) interact with neighboring genes to regulate the development and function of essential nerve cells. Scientists at the University of Bath led the study.
Despite the prevalence of genes coding for lncRNA in the genome (estimates range from 18,000-60,000 lncRNA genes in the human genome compared to 20,000 protein-coding genes), these segments of DNA were previously written off as junk precisely because the information contained within them does not result in the production of a protein. However, it is now evident that some lncRNAs are anything but trash, and these might end up being crucial in helping those with severe nerve damage regain their physical abilities.
A subset of lncRNA genes are co-expressed in the brain with neighboring genes that code for proteins involved in gene expression regulation, even though the function of the majority of lncRNA genes is still unknown. In other words, genes for these lncRNAs and their protein-coding neighbors work as a pair. Together, they control how vital nerve cells form and function, notably in the brain throughout embryonic development and early life.
The regulatory pathway involved in controlling the levels of one of these gene pairs is described in the new study. Their location and quantity in the genome need to be carefully coordinated, as does the timing of their activity.
“We previously defined one of the most profound functions for lncRNA in the brain and our new study identifies an important signaling pathway that acts to coordinate the expression of this lncRNA and the key protein coding gene that it is paired with,” explains Dr. Keith Vance, lead author of the study from the Department of Biology & Biochemistry at Bath.
“This new research takes us closer to understanding the basic biology of nerve cells and how they are produced. Regenerative medicine is the end-game and with further research we hope to develop a deeper understanding of how lncRNA genes operate in the brain.”
“This knowledge could be important for scientists looking for ways to replace defective neurons and restore nerve function – for instance in people who have had strokes,” explains Vance.
Reference: “Chromatin interaction maps identify Wnt responsive cis-regulatory elements coordinating Paupar-Pax6 expression in neuronal cells” by Ioanna Pavlaki, Michael Shapiro, Giuseppina Pisignano, Stephanie M. E. Jones, Jelena Telenius, Silvia Muñoz-Descalzo, Robert J. Williams, Jim R. Hughes and Keith W. Vance, 16 June 2022, PLOS Genetics.
DOI: 10.1371/journal.pgen.1010230
The research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and is published today in PLOS Genetics.