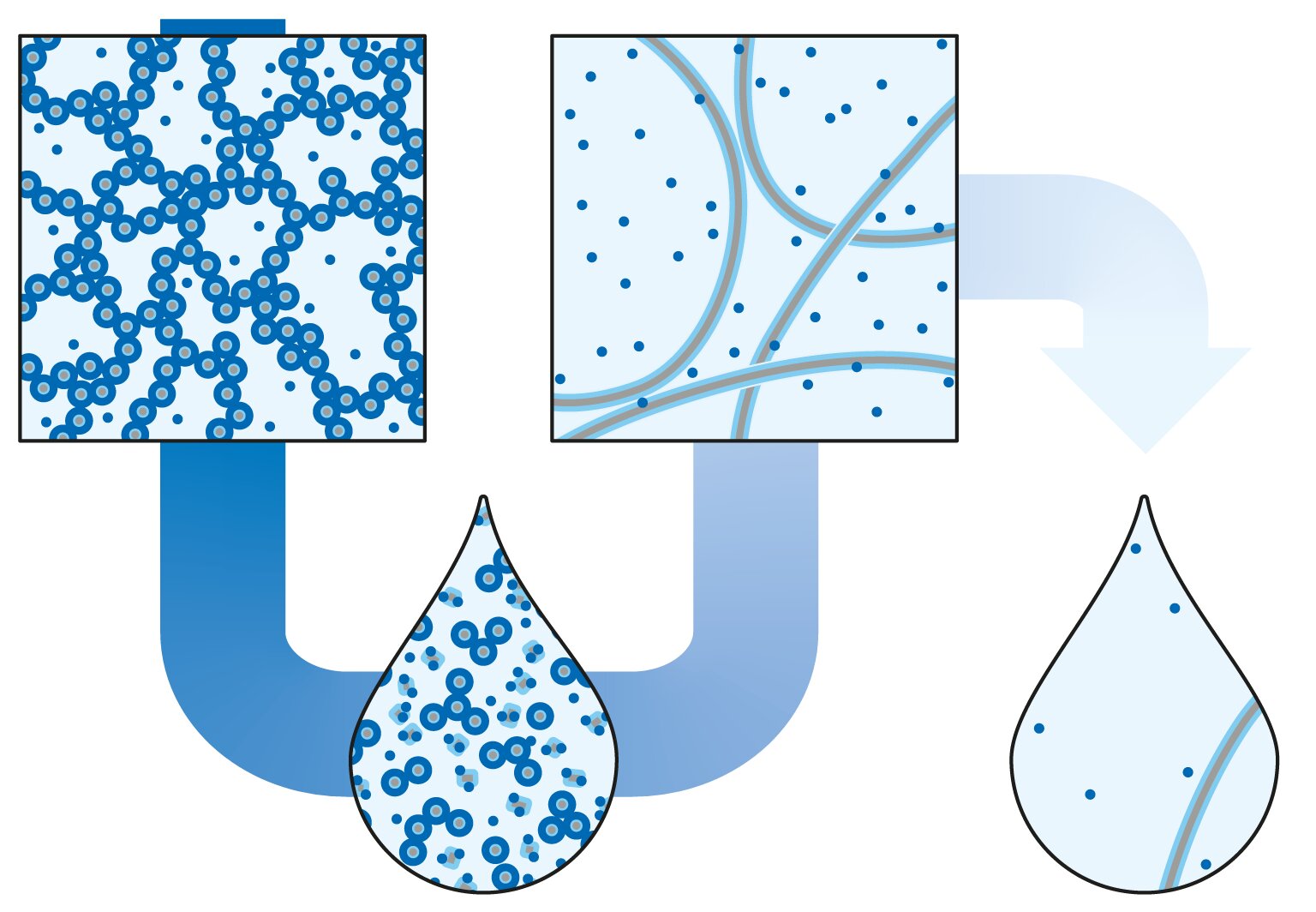
In Science, TU/e researchers have published their study on new phase transitions of solutions and gels in water, which seem to go against the basic principles of chemistry, and which they discovered by accident.
In chemistry, a hydrogel changes to a liquid by diluting it with water. For the reverse transition, you increase the hydrogel concentration. However, TU/e researchers led by Bert Meijer accidentally discovered that their liquid solution turned into a hydrogel when diluted. This phenomenon hadn’t been researched or described before and could have consequences in many areas in chemistry and biology.
The research focuses on the formation of certain hydrogels. This means that it starts with an aqueous solution of, in this case, two substances (a surfactant and a monomer). The research shows that a gel is formed at a specific ratio of these two substances in water. This gel is formed by long, supramolecular networks composed of both substances. The amounts of these substances in water (the concentrations) also determine where the phase transition of the gel formation is located. When decreasing the concentration without changing the ratio between the two components, the gel dissolves and becomes liquid. So far, this is familiar territory.
What is extraordinary, however, is that if the solution is diluted even further, a gel once again forms. Other supramolecular structures now form and it becomes a hydrogel again. And if it is then diluted even further, it becomes a liquid again. The paper carefully examined what the correct proportions of the active substances should be and at which concentrations the phase transitions take place. These transitions are also fully reversible. If concentrations are increased, the transitions from liquid to gel to liquid to gel occur at the same points. This phenomenon should be present in other fields, such as biology, but has never been researched and documented before.
Discovered by accident
This so-called dilution-induced self-assembly has been the subject of research in Bert Meijer’s research group for about ten years. However, it was difficult to achieve these transitions to form a solid and to get it working in aqueous solutions. But Jesús Mosquera and Cyprien Muller accidentally discovered in October 2019 that their liquid mixture of two components became a hydrogel when diluted.
“It is very valuable to show that what you learn in your first chemistry lessons does not always hold. When diluting, not all gels and solutions become liquids by definition,” says Meijer.
Lu Su, lead author of the Science paper, was immediately intrigued by that result. “It was a special, accidental finding. And I immediately saw more possibilities. What if we were able to demonstrate a double transition? So from a gel to a liquid, back to a gel and back to a liquid just by adding more water.”
“I have to be honest, when Jesús and Cyprien started their research, I was not immediately convinced that we had something special on our hands,” says Meijer. “But as a group leader, you have to support and trust young researchers when they start something they are excited about and you don’t yet see it yourself. As it was a new area for us, my intuition told me to be positive. And that’s the funny thing about research. Often, what you design in advance doesn’t work, but you can discover the unforeseen by accident. That is why I have supported them from the start in setting up and developing their research.”
Lockdowns
The timing of the finding is important to mention because not long afterward, the whole world was faced with lockdowns and the TU/e labs also closed. That gave Su a lot of time to think about how she could tackle this with her colleagues after Jesús and Cyprien left the Netherlands to pursue careers abroad. “During Teams meetings, we brainstormed with the group how we could set up the experiments. Together, we came up with a good setup for additional experiments.”
So when the labs reopened in the summer of 2020, they immediately began to explore their ideas. “And it turned out to be right!” Su says. “Within a month, we had done the basics of our experiments and demonstrated the hydrogel-solution-hydrogel-solution (gel-sol-gel-sol) transitions.”
In the fall, the researchers jointly wrote the first draft of their article, but the work was not yet finished. “When you find something special, you want to make sure that your interpretations are correct. That is why I encouraged them to invest in ensuring reproducibility and broadening the scope,” says Meijer. A new set of experiments was set up by Su. Unfortunately, because of her pregnancy, she was unable to perform them in the chemical lab herself, but colleagues were able to take over from her. Experiments were repeated several times to get even more reliable results.
According to Meijer, it is difficult to say how extensive the effects of their discovery will be, but it is certain that it will have a major impact on chemistry and biology. “Such hydrogels may be good solutions for existing challenges, such as for the cultivation of stem cells. In the gel, the cells can safely divide into three dimensions and, once there are enough, you dilute the solution and the cells can be used right away. That is, for example, research that we are currently doing together with the group of Patricia Dankers. The trick is, of course, to find the right substances that exhibit this behavior but do not react to the cell walls or stick to them. In this way, our research leads to other research.”
Evidence found for existence of two forms of liquid water
Lu Su et al, Dilution-induced gel-sol-gel-sol transitions by competitive supramolecular pathways in water, Science (2022). DOI: 10.1126/science.abn3438. www.science.org/doi/10.1126/science.abn3438
Matthew J. Webber, Less is more when forming gels by dilution, Science (2022). DOI: 10.1126/science.abo7656 , www.science.org/doi/10.1126/science.abo7656
Eindhoven University of Technology
Citation:
Chemists find a contrary effect: How diluting with water makes a solution firm (2022, July 7)
retrieved 8 July 2022
from https://phys.org/news/2022-07-chemists-contrary-effect-diluting-solution.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.