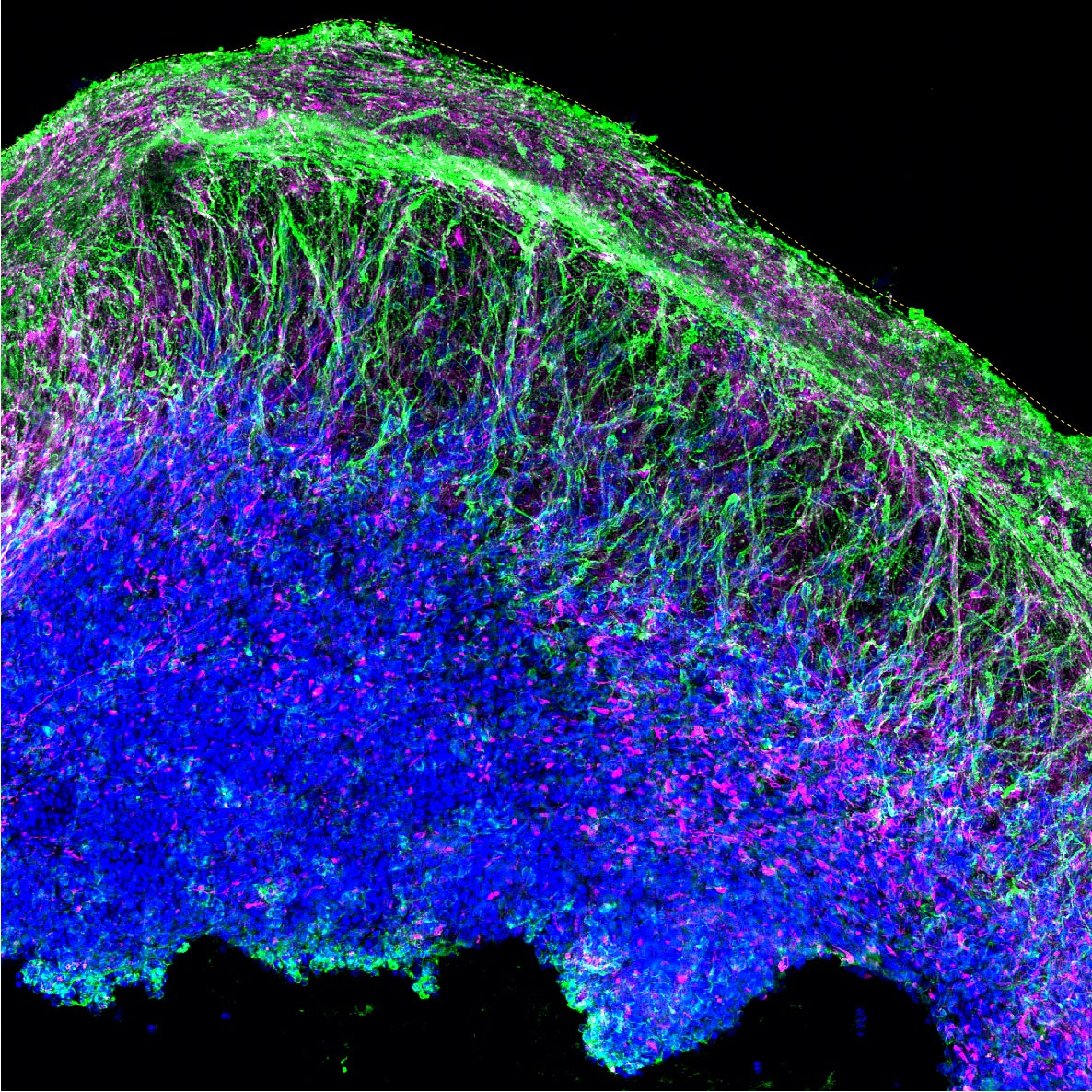

The organoids contained an array of neural and other cell types found in the cerebral cortex, the outermost layer of the brain involved in language, emotion, reasoning, and other high-level mental processes. Credit: Yueqi Wang
The structures are reminiscent of one wrinkle of a human brain at 15 to 19 weeks post-conception.
Whatever you do, don’t call them “mini-brains,” say scientists at University of Utah Health. Regardless of what they are called, the seed-sized organoids—which are grown from human cells in the lab—provide insights into the brain and uncover differences that may contribute to autism in some people.
“We used to think it would be too difficult to model the organization of cells in the brain,” says Alex Shcheglovitov, PhD, assistant professor of neurobiology at U of U Health. “But these organoids self-organize. Within a few months, we see layers of cells that are reminiscent of the cerebral cortex in the human brain.”
The research describing the organoids and their potential for understanding neural diseases will be published today (October 6) in the journal Nature Communications with Shcheglovitov as senior author and Yueqi Wang, PhD, a former graduate student in his lab, as lead author. They carried out the research with postdoctoral scientist Simone Chiola, PhD, and other collaborators at the University of Utah, Harvard University, University of Milan, and Montana State University.

Sesame seed-sized brain-like organoids are grown in the lab from human cells. They are providing insights into the brain and uncovering differences that may contribute to autism in some people. Credit: Trevor Tanner
Investigating autism
Having the ability to model aspects of the brain in this way gives scientists a glimpse into the inner workings of a living organ that is otherwise nearly impossible to access. And since the organoids grow in a dish, they can be tested experimentally in ways that a brain cannot.
Shcheglovitov’s team used an innovative process to investigate the effects of a genetic abnormality associated with autism spectrum disorder and human brain development. They found that organoids engineered to have lower levels of the gene, called SHANK3, had distinct features.

Single neural rosette-derived organoids develop multiple brain cell types and have an organization and neural activity never seen before in models of this kind. Credit: Trevor Tanner
Even though the autism organoid model appeared normal, some cells did not function properly:
- Neurons were hyperactive, firing more often in response to stimuli,
- Other signs indicated neurons may not efficiently pass along signals to other neurons,
- Specific molecular pathways that cause cells to adhere to one another were disrupted.
According to the authors, these findings are helping to uncover the cellular and molecular causes of symptoms associated with autism. They also demonstrate that the lab-grown organoids will be valuable for gaining a better understanding of the brain, how it develops, and what goes wrong during disease.
“One goal is to use brain organoids to test drugs or other interventions to reverse or treat disorders,” says Jan Kubanek, PhD, a co-author on the study and an assistant professor of biomedical engineering at the U.

Simone Chiola, PhD, picks radial structures called neural rosettes that have formed from human stem cells. Over months, the structures from become spheroid organoids that model aspects of the human brain. Credit: Nika Romero
Building a better brain model
Scientists have long searched for suitable models for the human brain. Lab-grown organoids are not new, but previous versions did not develop in a reproducible way, making experiments difficult to interpret.
To create an improved model, Shcheglovitov’s team took cues from how the brain develops normally. The researchers prompted human stem cells to become neuroepithelial cells, a specific stem cell type that forms self-organized structures, called neural rosettes, in a dish. Over the course of months, these structures coalesced into spheres and increased in size and complexity at a rate similar to the developing brain in a growing fetus.
After five months in the lab, the organoids were reminiscent of “one wrinkle of a human brain” at 15 to 19 weeks post-conception, Shcheglovitov says. The structures contained an array of neural and other cell types found in the cerebral cortex, the outermost layer of the brain involved in language, emotion, reasoning, and other high-level mental processes.
Like a human embryo, organoids self-organized in a predictable fashion, forming neural networks that pulsated with oscillatory electrical rhythms and generated diverse electrical signals characteristic of a variety of different kinds of mature brain cells.
“These organoids had patterns of electrophysiological activity that resembled actual activity in the brain. I didn’t expect that,” Kubanek says. “This new approach models most major cell types and in functionally meaningful ways.”
Shcheglovitov explains that these organoids, which more reliably reflect intricate structures in the cortex, will allow scientists to study how specific types of cells in the brain arise and work together to perform more complex functions.
“We’re beginning to understand how complex neural structures in the human brain arise from simple progenitors,” Wang says. “And we’re able to measure disease-related phenotypes using 3D organoids that are derived from stem cells containing genetic mutations.”
He adds that by using the organoids, researchers will be able to better investigate what happens at the earliest stages of neurological conditions, before symptoms develop.
Reference: “Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes” 6 October 2022, Nature Communications.
DOI: 10.1038/s41467-022-33364-z
Funding: NIH/National Institute of Mental Health, NIH/National Institute of Neurological Disorders and Stroke, NIH/National Institute of Neurological Disorders and Stroke
Support for the work came from the National Institutes of Health, Brain Research Foundation, Brain and Behavior Research Foundation, Whitehall Foundation, University of Utah Neuroscience Initiative, and University of Utah Genome Project Initiative.

